February 2024
Guidelines and Recommendations for Targeted Neonatal Echocardiography and Cardiac Point-of-Care Ultrasound in the Neonatal Intensive Care Unit: An Update from the American Society of Echocardiography
Guidelines and Recommendations for Targeted Neonatal Echocardiography and Cardiac Point-of-Care Ultrasound in the Neonatal Intensive Care Unit: An Update from the American Society of Echocardiography
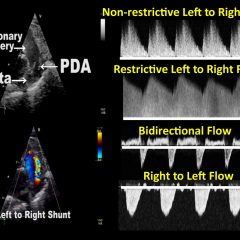
Targeted neonatal echocardiography (TNE) involves the use of comprehensive echocardiography to appraise cardiovascular physiology and neonatal hemodynamics to enhance diagnostic and therapeutic precision in
the neonatal intensive care unit. Since the last publication of guidelines for TNE in 2011, the field has matured through the development of formalized neonatal hemodynamics fellowships, clinical programs, and the expansion of scientific knowledge to further enhance clinical care. The most common indications for TNE include adjudication of hemodynamic significance of a patent ductus arteriosus, evaluation of acute and chronic pulmonary hypertension, evaluation of right and left ventricular systolic and/or diastolic function, and screening for pericardial effusions and/or malpositioned central catheters. Neonatal cardiac point-of-care ultrasound (cPOCUS) is a limited cardiovascular evaluation which may include line tip evaluation, identification of pericardial effusion and differentiation of hypovolemia from severe impairment in myocardial contractility in the hemodynamically unstable neonate. This document is the product of an American Society of Echocardiography task force composed of representatives from neonatology-hemodynamics, pediatric cardiology, pediatric cardiac sonography, and neonatology-cPOCUS. This document provides (1) guidance on the purpose and rationale for both TNE and cPOCUS, (2) an overview of the components of a standard TNE and cPOCUS evaluation, (3) disease and/or clinical scenario–based indications for TNE, (4) training and competency-based evaluative requirements for both TNE and cPOCUS, and (5) components of quality assurance. NOTE: This guideline replaces the 2011 TNE guideline and has been expanded to provide recommendations for cardiac point-of-care ultrasound (cPOCUS). NOTE: This guideline had a Correction released 4/25/2024, https://doi.org/10.1016/j.echo.2024.04.006, the pdf has been updated.
Chair(s)
- McNamara, Patrick
