ASE Consensus Statement on Hypersensitivity Reactions to Ultrasound Enhancing Agents

In an effort to help improve quality and encourage the appropriate use of ultrasound contrast, ASE established the ContrastZone website to provide the cardiovascular ultrasound community with a central place on the web to find both basic and advanced information about Ultrasound Enhancing Agents (UEA), also known as ultrasound contrast. This site includes links to recent ASE guidelines and standards, how-to videos, reimbursement information, and success stories from busy labs on how to incorporate UEAs into everyday practice to improve overall quality. Explore the menu to learn more!
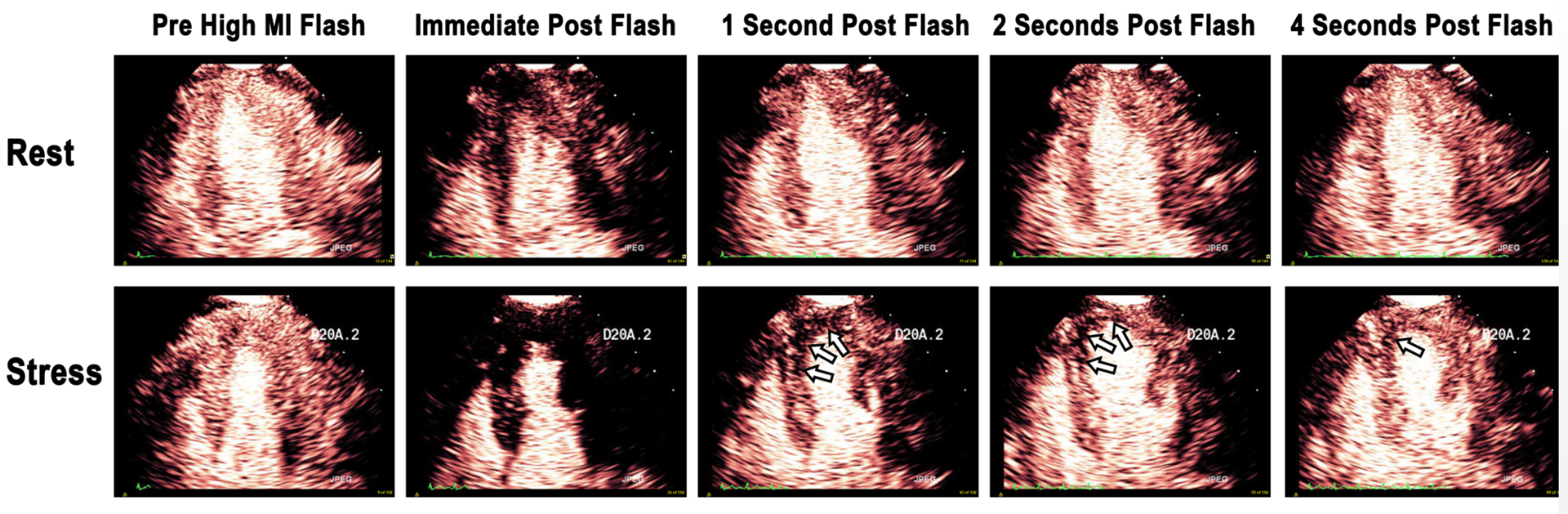
An example of a stress-induced perfusion defect in the left anterior descending coronary artery (LAD) territory (arrows). Note that end-systolic replenishment within the LAD territory in the apical four-chamber window is normal under resting conditions but delayed in the LAD territory (arrows) during dobutamine stress imaging. Figure 13 from “Clinical Applications of Ultrasonic Enhancing Agents in Echocardiography: 2018 American Society of Echocardiography Guidelines Update”, JASE, March 2018
